Non-Hazardous Pharmaceutical Waste
For most facilities, greater than 90% of your pharmaceutical waste will be non-hazardous pharmaceutical waste. Now non-hazardous doesn’t mean it’s just trash- in industry terms, it just means that it is state-regulated versus federally-regulated. However, this is good news for two reasons. First, it makes segregating your waste much easier. Second, average disposal costs are significantly decreased for non-hazardous pharmaceutical waste.
Examples of Non-Hazardous Pharmaceuticals
- OTC Medications
- Non-DEA Controlled/Non-RCRA Hazardous Prescription Medications
- Hormones
- Allergy Medications
- Ointment/Cream medications
- Vitamins & Supplements
- Most Vaccines
RCRA Hazardous Pharmaceutical Waste
The next and most regulated category of waste is federally regulated RCRA Hazardous pharmaceutical waste and DOT Hazardous materials. RCRA stands for Research Conservation & Recovery Act. This is the set of federal laws that govern hazardous waste management at a national level and are enforced by the EPA.
DOT stands for the Department of Transportation. They too have a list of pharmaceuticals they consider hazardous materials but some do not appear on the RCRA list.
Fortunately, there are only a relative handful of medications on the federal RCRA list or the Department of Transportation’s (DOT) table of hazardous materials. To simplify it even further for most, many of the listed items are only used by specialized facilities like hospitals or pharmacies.
At Approved, regardless of size, we will implement a simple, easy to follow program that fits the needs of your facility.
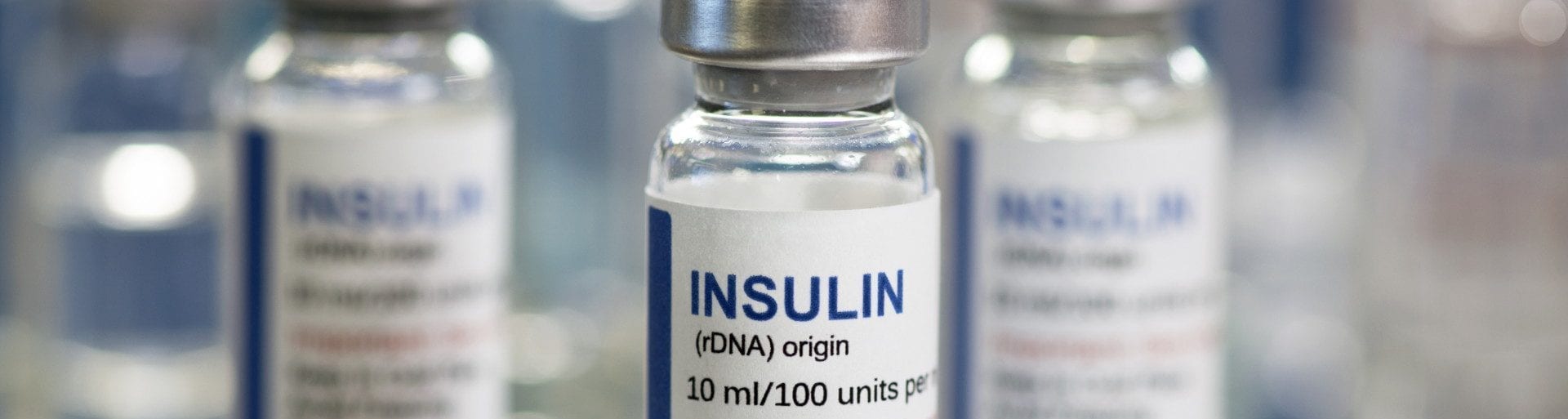
Examples of U & D Listed RCRA Hazardous Waste Pharmaceuticals
- Cyclophosphamide
- Mitomycin
- Insulin
- Phenol
- Selenium Sulfide
- Mercury & Silver containing medications
- Lindane
Examples of P-LISTED RCRA Hazardous Waste Pharmaceuticals
- Coumadin/Warfarin
- Nicotine (Deregulated in several states, consult with one of our experts)
- Epinephrine & Nitroglycerin (CT only)
Your specific circumstances and the amount of waste you generate will ultimately determine your generator status and the number of containers you will need on-site. Many facilities will be able to combine these two sub-categories.
Examples of RCRA Hazardous AEROSOL Waste Pharmaceuticals
All Aerosols and Inhalers fall under this category. Please note that an aerosol will always be in a pressurized metal canister. If it is a plastic inhalation device, it can be managed as non-hazardous pharmaceutical waste. Other examples include:
- Albuterol rescue inhalers
- Symbicort
- Ethyl Chloride
- Atrovent
- Dermoplast
Upon implementation, ASWH will provide all required, properly labeled DOT approved waste collection containers. At the time of disposal, one of our technicians will ensure that your waste is properly packaged and provide a waste tracking manifest for your records. Replacement containers will be provided by ASWH as needed.
New EPA Final Rule on Pharmaceutical Waste Management
CFR 49 Part 266 Sub-part P
On August 21, 2019, The Federal Environmental Protection Agency enacted a new final rule pertaining to pharmaceutical waste management and disposal. If your facility generates pharmaceutical waste, the new regulations, CFR 49 Part 266 Subpart P, will likely affect your operations if they have not done so already.
Under the new regulations, an immediate nationwide ban on sewering or flushing hazardous pharmaceuticals is now in effect. It is of the utmost importance to ensure that you are prepared to comply with all the new regulations.
Depending on your state’s individual government structure, a state has until July 2021 or July 2022 to adopt the entire set of new regulations. However, these regulations are not optional- states must adopt the new regulations because they are more stringent. A state can only choose to opt-out of less stringent regulations. Many states have immediately adopted the new regulations.
New Requirements for Healthcare Facilities Under Part 266 Subpart P
- Healthcare facilities will need to determine their hazardous waste generator status.
- Provide a one-time notification to the EPA regional administrator.
- Properly manage their hazardous pharmaceutical waste going forward.
Benefits of the New Regulations
Hazardous pharmaceuticals will no longer count toward generator status. As a result, many generators will be able to reduce their generator status or, at a minimum, remain a VSQG even if the waste volume increases.
- The limitations on shipping P-listed pharmaceuticals like Coumadin will be eliminated as they will no longer count toward generator status. The need for VSQG and SQG generators to segregate the select few P-listed medications is no longer required.
- Satellite storage limitations are reduced, made more user-friendly, and made more cost-effective.
- Hazardous pharmaceuticals can be stored up to 365 days at the site of the generator regardless of generator status.
- Training & Reporting requirements are greatly reduced or eliminated.
- OTC Nicotine replacement products are no longer considered hazardous waste and can be discarded as non-hazardous pharmaceutical waste when unused or as regular trash when used.
- Medications considered both hazardous and DEA controlled substances are now regulated ONLY as DEA controlled substances.
As a healthcare provider, it is your obligation to comply with all current regulations and any new ones that may arise. At Approved, we stay up to date with changing regulations so you can focus on what’s important- your business. Let us implement a simple and cost-effective pharmaceutical waste management program today so you don’t find yourself in an unfortunate situation with a regulator tomorrow.